Who List Of Covid Vaccines
The arrival of Covid-19 vaccines promises a return to more normal life and has created a global market worth tens of billions of dollars in annual sales for some pharmaceutical companies. Vaccines safety The world is in the midst of a COVID-19 pandemic.
Chart The Countries Dominating Covid 19 Vaccine Production Statista
WHO today listed the Sinopharm COVID-19 vaccine for emergency use giving the green light for this vaccine to be rolled out globally.
Who list of covid vaccines. Status of COVID-19 Vaccines within WHO EULPQ evaluation process. Heres a list of COVID vaccines approved by WHO. AstraZeneca COVID-19 vaccine Novavax COVID-19 vaccine Learn more about US.
COVID-19 vaccine clinical trials including vaccines in earlier stages of development by visiting clinicaltrialsgov. As of February 27 2021 large-scale Phase 3 clinical trials are in progress or being planned for two COVID-19 vaccines in the United States. A full list of our country-specific sources is available at the bottom of this page and we also answer frequently-asked questions there.
It reflects those vaccines which following a selection based on immunization priorities set by relevant UN agencies and WHO have been submitted to WHO for evaluation by interested parties and have at the time of evaluation and site inspection been found to meet the aforementioned WHO-recommended standards and. The vaccination drive for Covid 19 has been started across the globe. AstraZeneca anticipates producing 3 billion doses in 2021 PfizerBioNTech 13 billion doses and Sputnik V Sinopharm Sinovac and Janssen 1.
Below are the top companies which are involved in the development and manufacturing of Covid vaccines. COVID-19s caused virus SARS-CoV-2 severe acute respiratory syndrome coronavirus 2 was isolated in late 2019. Vaccines have been developed and manufactured by a lot of companies around the world.
Vaccines Guidance Document 16 February 2021. Equitable access to safe and effective vaccines is critical to ending the COVID-19 pandemic so it is hugely encouraging to see so many vaccines proving and going into development. Roadmap for evaluation of AstraZeneca AZD1222 Vaccine against Covid-19.
WHO operational tool for efficient and effective lot release of SARS-CoV-2 COVID-19 vaccines. Vaccines Guidance Document 15 July 2021. 19 January 2021.
Roadmap for Evaluation of Janssen Ad26COV2-S recombinant vaccine against COVID-19. 30 October 2020. WHO is working tirelessly with partners to develop manufacture and deploy safe and effective vaccines.
Addendum evaluation of modified COVID-19 vaccines. Use of Emergency Use Listing procedure for vaccines against COVID-19 QA. WHO Certified COVID Vaccines WHO has approved Covishield which is the local name for the Oxford-AstraZeneca vaccine that is being manufactured locally by the Serum Institute of India.
As WHO and partners work together on the response -- tracking the pandemic advising on critical interventions distributing vital medical supplies to those in need--- they are racing to develop and deploy safe and effective vaccines. Considerations for the Assessment of COVID-19 Vaccines for Listing by WHO. Manufacturer Name of Vaccine NRA of Record Platform EOI accepted Pre-submission meeting held Dossier accepted for review Status of assessment Anticipated decision date 1.
Its genetic sequence was published on 11 January 2020 triggering an urgent international response to prepare for an outbreak and hasten development of a preventive COVID-19 vaccine. Status of COVID-19 Vaccines within WHO EULPQ evaluation process. Pfizer Astra Zeneca Serum Institute of India Bharath Biotech Moderna BioNTech Novavax.
World Health Organization Coronavirus disease situation dashboard presents official daily counts of COVID-19 cases and deaths worldwide along with vaccination rates and other vaccination data while providing a hub to other resources. Considerations for the assessment of COVID-19 vaccines for listing by WHO. The Janssen vaccine leverages the AdVac vaccine platform to engineer a common cold virus to carry genetic instructions into the arm cells where they construct a replica of the coronavirus spike triggering the immune system.
480 billion doses of COVID19 vaccine have been administered worldwide based on official reports from national public health agencies. The reported side effects from the vaccines include migraines anaphylaxis seizures paralysis and sudden death. The population estimates we use to calculate per-capita metrics are all based on the last revision of the United Nations World Population Prospects.
Our vaccination dataset uses the most recent official numbers from governments and health ministries worldwide. In fact its one of just seven vaccines that the WHO has approved for inclusion on the EUL. Manufacturer WHO EUL holder Name of Vaccine NRA of Record Platform EOI accepted Pre-submission meeting held Dossier accepted for review Status of assessment Decision date 1.
Coronavirus disease COVID-19. Experts believe long-term effects from the gene therapy may include prion diseases such as Alzheimers cancers kidney diseases and microvascular injuries to the brain liver and. The list is not an exhaustive list of vaccines used to immunize humans.
The one-off vaccine was listed by the WHO for emergency use and COVAX roll-out on 12 March 2021. The Sinopharm vaccine is produced by Beijing Bio-Institute of Biological Products Co Ltd subsidiary of China National Biotec Group CNBG. Since 2020 vaccine development has been expedited via unprecedented collaboration in.
The Hushed Long-Term Risks of COVID-19 Vaccines. Product eligibility under the COVAX Facility. Interactive tools including maps epidemic curves and other charts and graphics with downloadable data allow users to track and explore the latest trends.
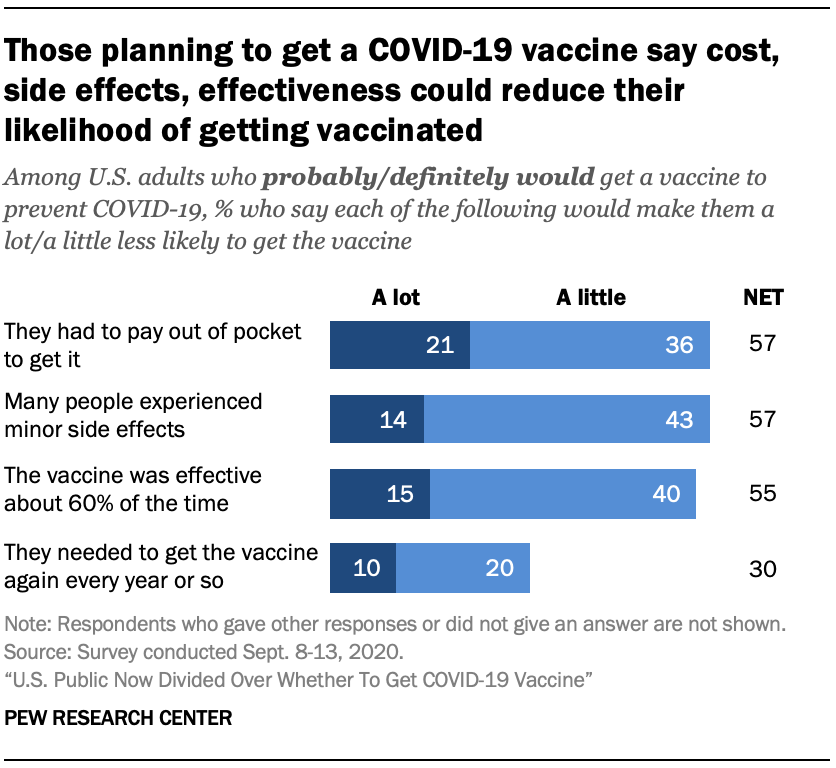
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
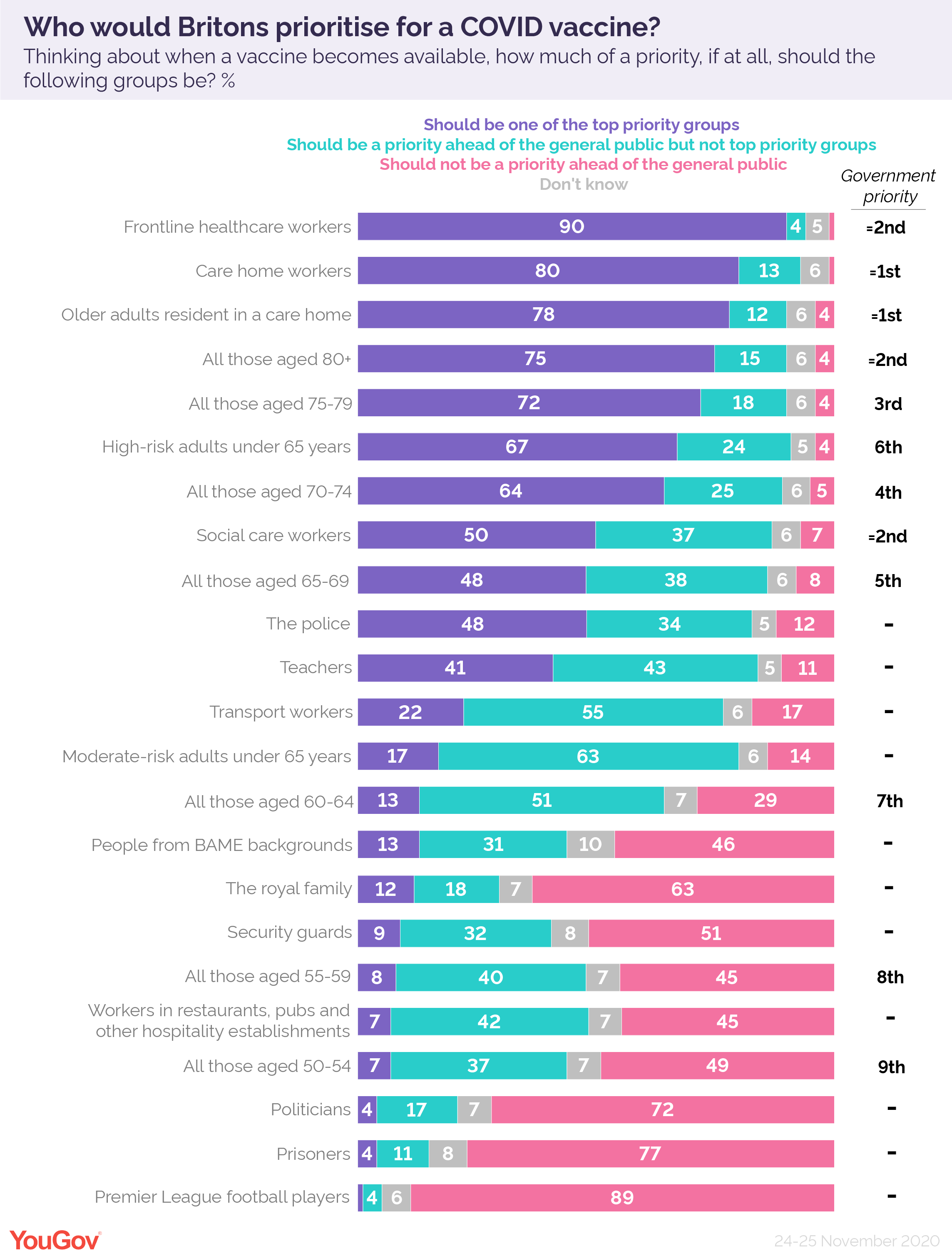
Who Should Be Prioritised For A Covid Vaccine Yougov
Chart Where Coronavirus Vaccines Will Be Produced Statista

Every Vaccine And Treatment In Development For Covid 19 So Far

A Comprehensive List Of All Covid 19 Vaccine Ingredients
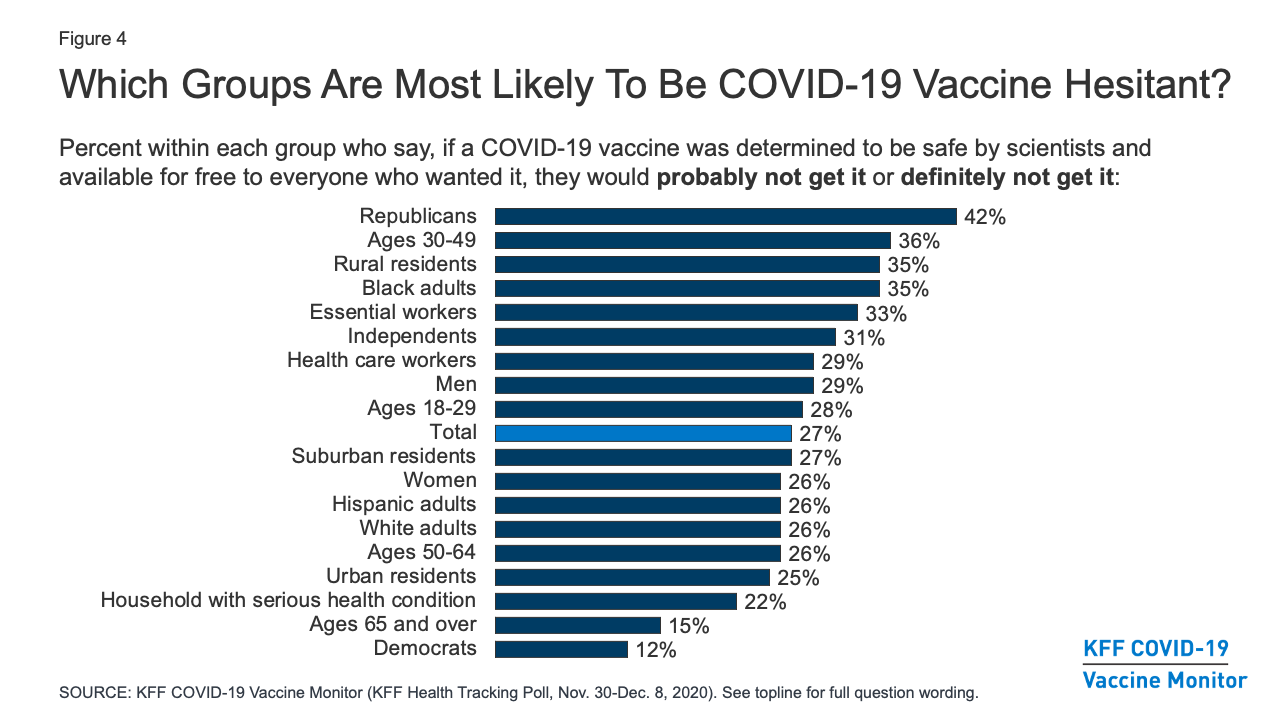
Kff Covid 19 Vaccine Monitor December 2020 Kff
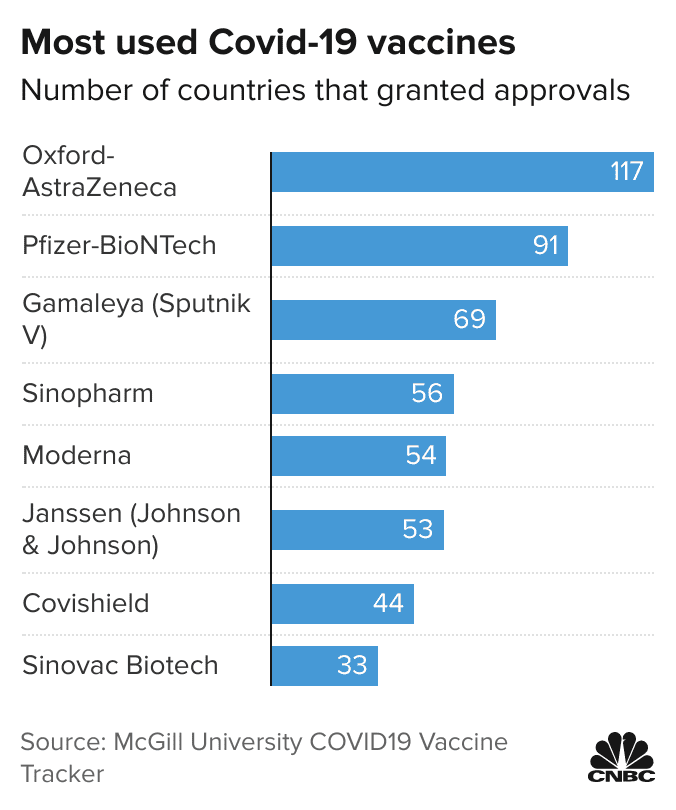
Five Vaccinated Countries With High Covid Rates Rely On China Vaccines
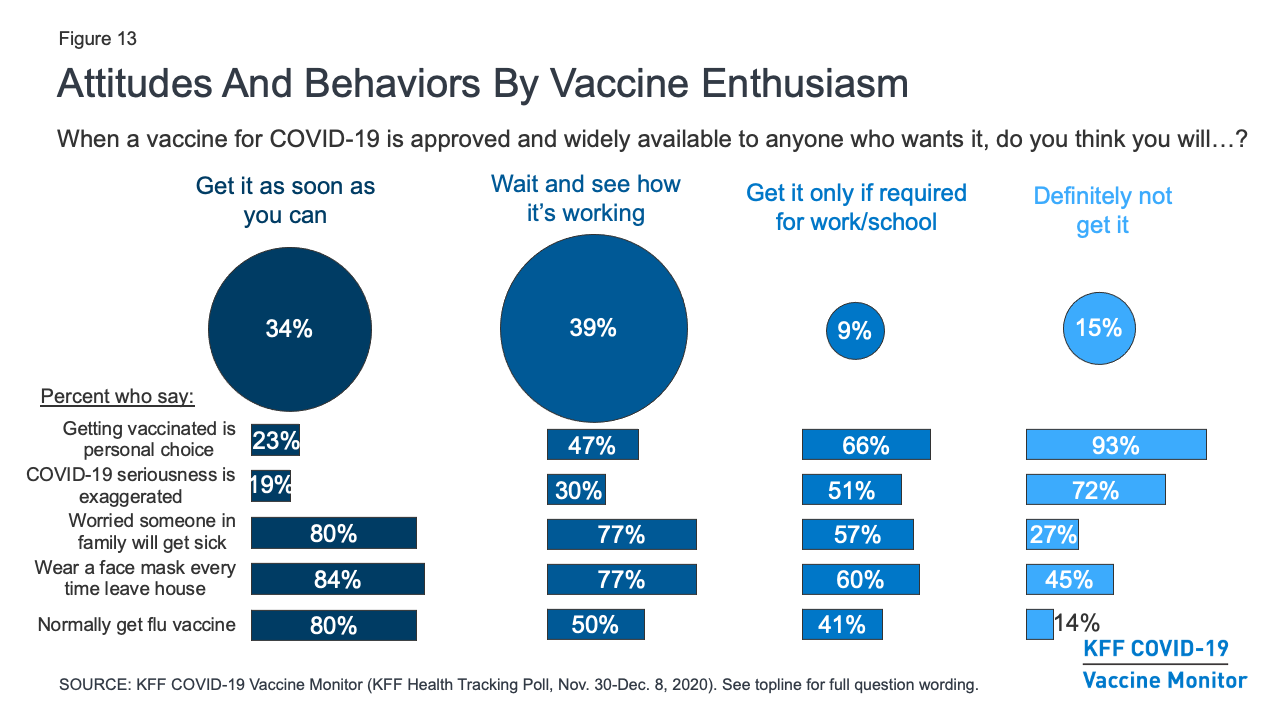
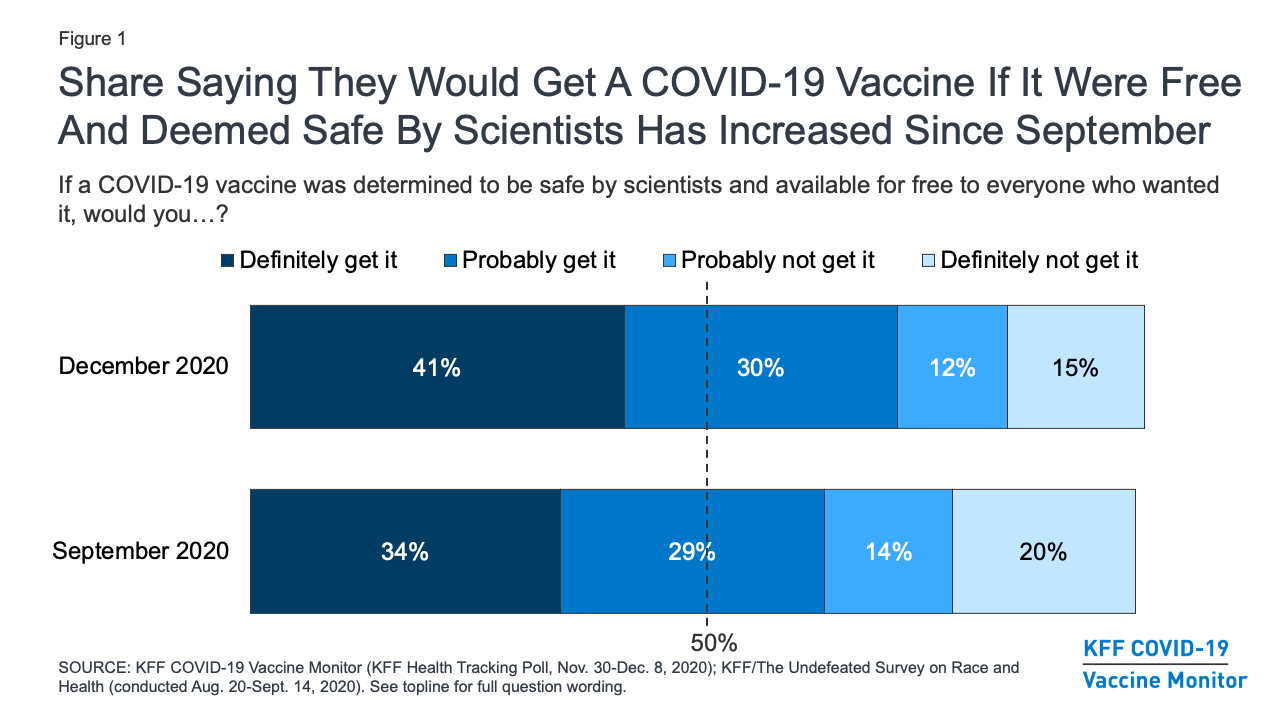
Kff Covid 19 Vaccine Monitor December 2020 Kff
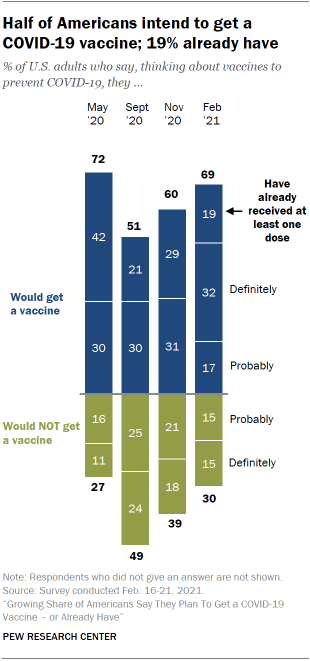
Growing Share Of Americans Say They Plan To Get A Covid 19 Vaccine Or Already Have Pew Research Center
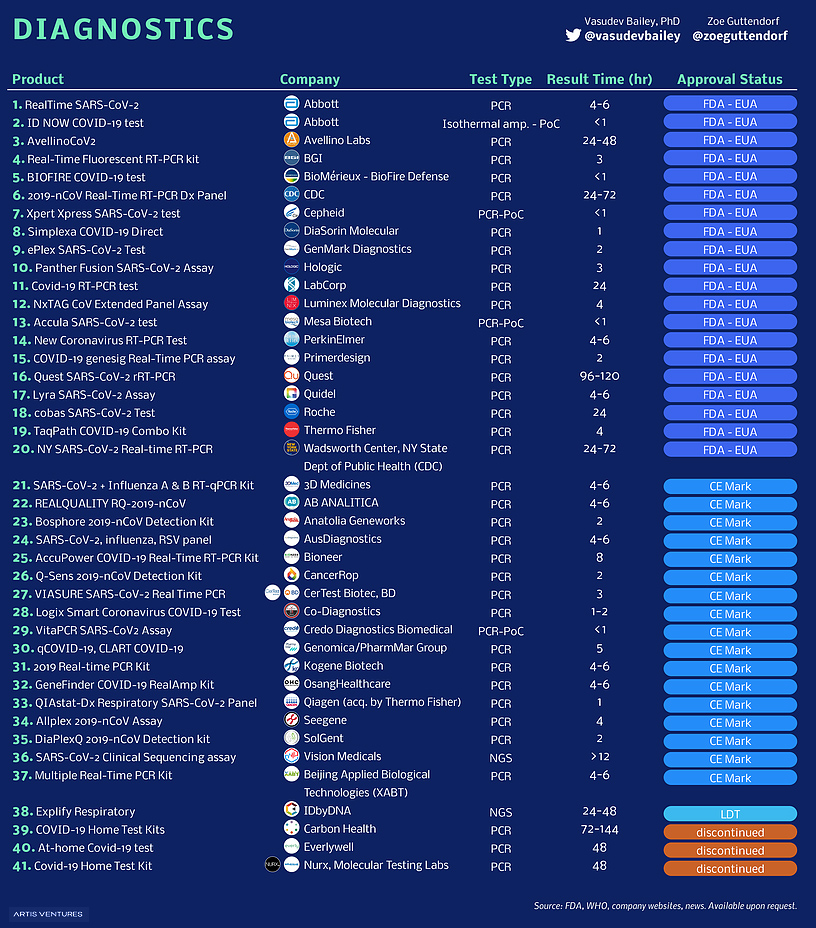
Every Vaccine And Treatment In Development For Covid 19 So Far

Covid 19 Vaccine Need To Know Fliers Posters And Graphics Mass Gov
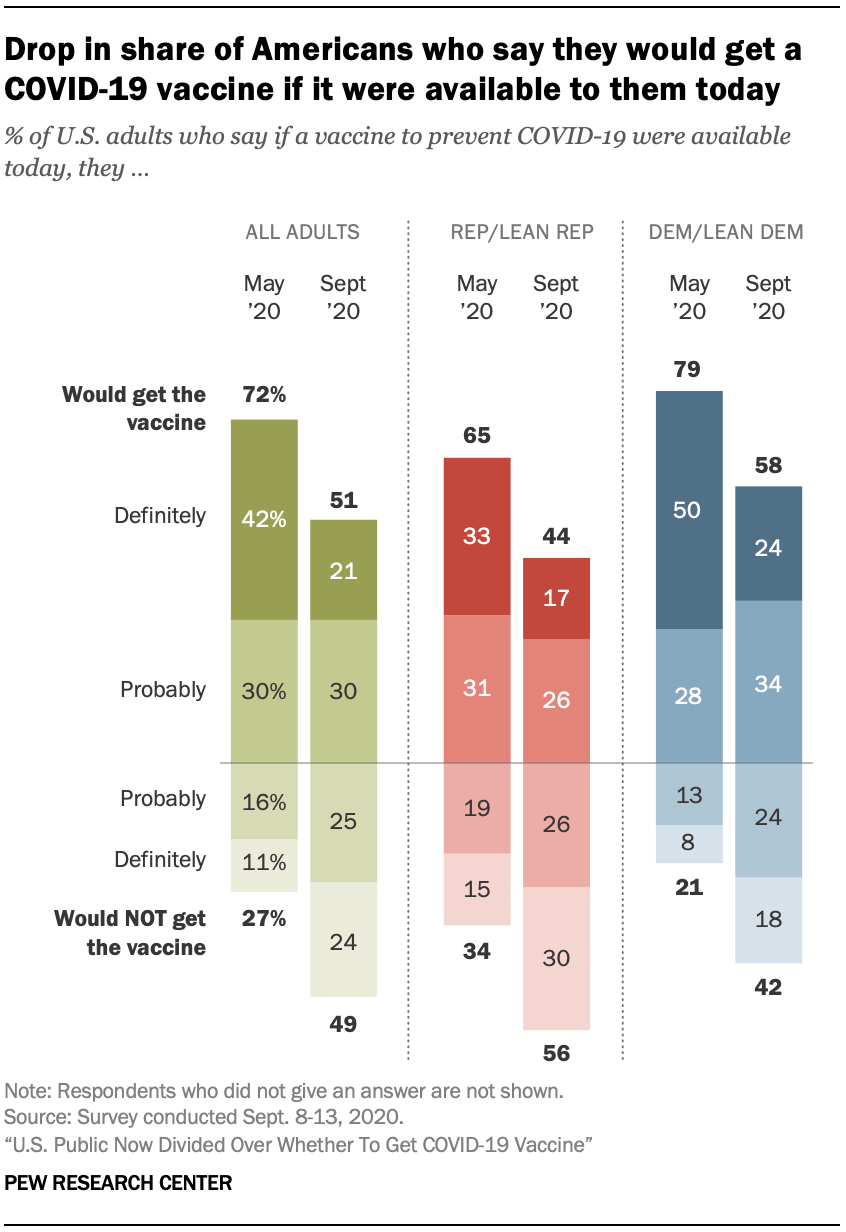
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
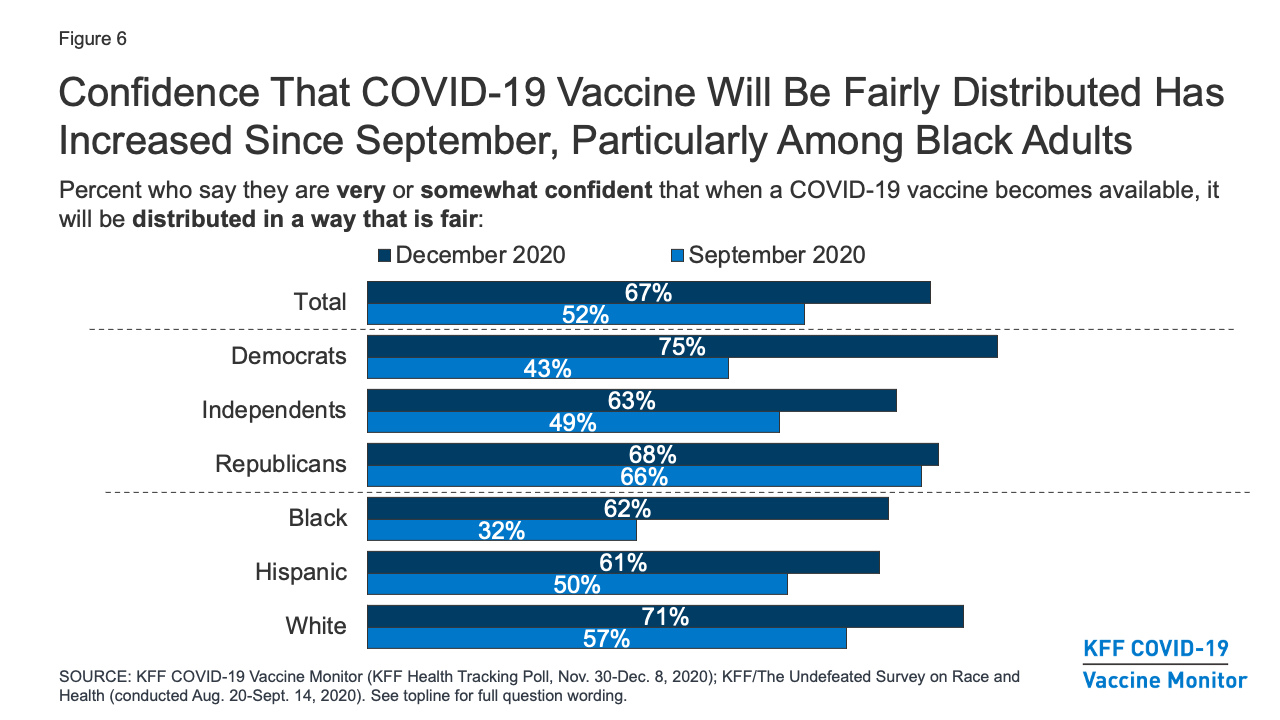
Kff Covid 19 Vaccine Monitor December 2020 Kff

Kff Covid 19 Vaccine Monitor December 2020 Kff
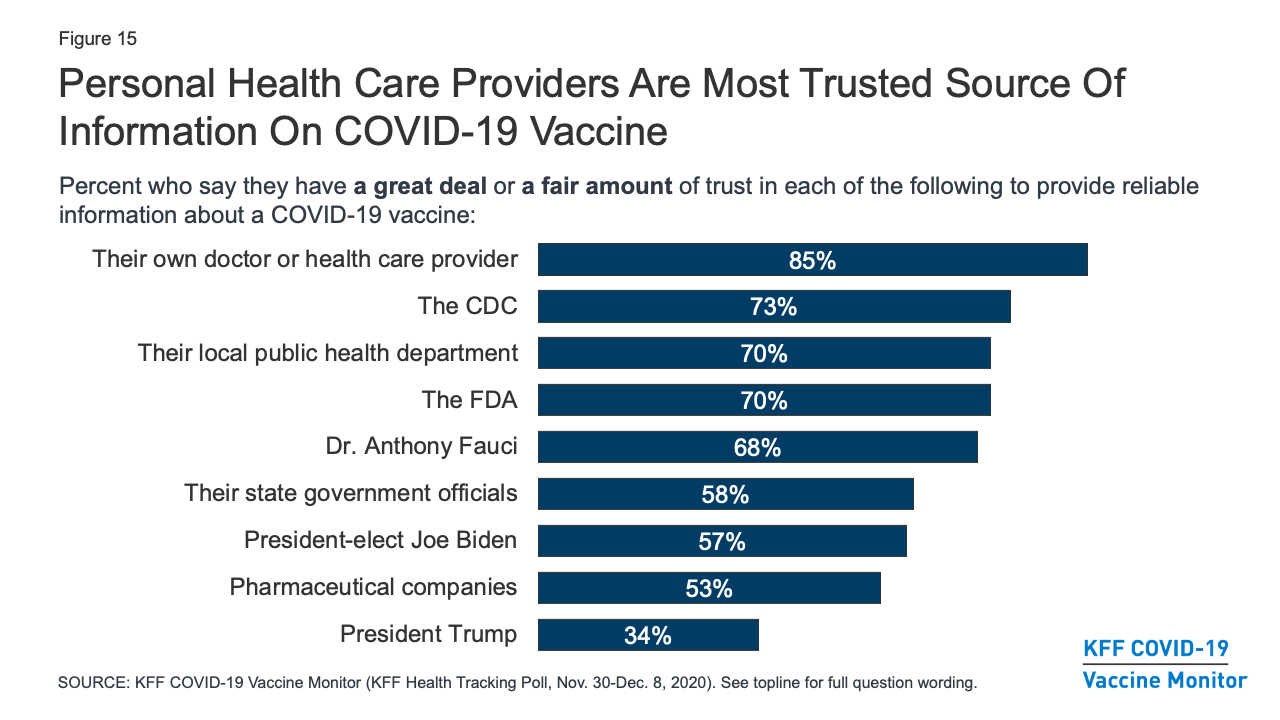
Kff Covid 19 Vaccine Monitor December 2020 Kff
Coronavirus Vaccine Efficacy Compared To Shots For Other Viruses

Explained Who Should Not Take The Coronavirus Vaccine Shots India News
Chart The Covid 19 Vaccination Race In Asia Statista

Coronavirus Covid 19 Vaccines And Vaccination Campaign Statista
Post a Comment for "Who List Of Covid Vaccines"